 Let's take a look at how semiconductor diodes are used to detect ionising radiation.
Let's take a look at how semiconductor diodes are used to detect ionising radiation.Within an inorganic solid, the atoms are typically arranged into a crystal lattice of some description. The electrons in the outermost shells of the atoms are called the valence electrons, and their energy levels make up the valence band. In a conductor, the electrons in the valence band are easily separated from their host atoms, and are free to migrate through the lattice. The energy levels of such electrons belong to the conduction band, and in a conductor there is no gap between the valence band and the conduction band. In the case of both an insulator and a semiconductor, there is a gap between the valence band and the conduction band, but in the case of a semiconductor it is a smaller gap, and electrons can be induced to jump across the gap.
When an ionising particle enters a semiconductor, it is capable of transferring its energy to electrons in the valence band, causing them to jump into the conduction band. Each time this occurs, a so-called electron-hole pair is created. That is, an electron is liberated from its lattice site, and becomes capable of moving freely through the lattice, and, in addition, the removal of an electron leaves a hole in the valence band. The hole is also capable of moving through the lattice structure, but whilst the electron moves through the conduction band, the hole moves through the valence band. Given a hole in the outermost shell of one atom, an electron from the outermost shell of a neighbouring atom is capable of popping across into that hole, with the result that the hole itself migrates in the opposite direction.
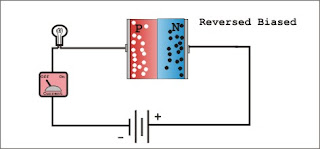
A common type of semiconductor used to detect ionising radiation is a silicon or germanium crystal. Ionising radiation creates electron-hole pairs in the crystal, and by applying an electric field across the crystal, the electrons and holes can be attracted towards an anode and a cathode, respectively. The accumulated charge can then be counted, and used to infer the energy of the radiation impinging upon the detector. Specifically, the semiconductor in such a detector is fabricated into a diode, a device in which two types of semiconductor material lie face-to-face across a junction, and a voltage is applied. The most popular type of semiconductor diode is a 'reverse biased p-n junction'.
A p-type semiconductor is doped with atoms that have less valence electrons than the normal number of covalent bonds with neighbours in the crystal lattice. These acceptor sites create vacant covalent bonds, which are filled by electrons that would otherwise be bound by normal covalent bonds elsewehere in the lattice. As a consequence, the acceptor impurities create holes in the valence band, and these holes are the majority charge carriers in the p-type semiconductor.
An n-type semiconductor is doped with atoms that have more valence electrons than required for the covalent bonds to the neighbouring atoms in the crystal lattice. The spare electrons at these donor sites are easily dislodged into the conduction band, without creating a corresponding hole in the valence band. As a consequence, free electrons are the majority charge carriers in an n-type semicondunctor.
When a p-type semiconductor and an-type semiconductor lie face-to-face across a junction, the respective charge carriers migrate across the junction some distance into the other material. Conduction electrons migrate from the n-type material into the p-type material, where they fill the holes in the p-type material. Conversely, holes from the p-type material migrate across the junction and recombine with electrons in the n-type material. The immobile donor sites in the n-type material have a net positive charge, and the immobile acceptor sites in the p-type material have a net negative charge, hence the result is that, within a certain distance either side of the junction, there is a net positive charge on the n side, and a net negative charge on the p side. The region over which this charge imbalance exists is called the depletion region, because the region becomes depleted of mobile charge carriers. Whilst this charge imbalance corresponds to a spontaneous voltage across
the junction, the voltage is small.

In the absence of an external voltage, the junction is referred to as an unbiased junction. An external forward bias voltage across the junction is one whose direction is opposite to that of the spontaneous voltage. A reverse bias voltage is one whose direction matches that of the natural potential difference across the depletion region. A forward bias voltage will result in a large current because the potential will attract the respective majority charge carriers across the junction, electrons from the n side and holes from the p side. In contrast, a reverse bias voltage will result in only a small current because the potential attracts the minority charge carriers across the junction, holes from the n side and electrons from the p side. Because there are relatively few mobile charges left in the depletion region, very little intrinsic current is created in the depletion region. A diode therefore functions as a rectifier, in the sense that, under the application of an external voltage, it permits the free flow of current in one direction, but presents a large resistance to its flow in the other. It is in the depletion region, with the application of a reverse bias voltage, that the semiconductor diode can function as an effective detector of ionising radiation. The electric field in the region is such that when any electron-hole pairs are created by ionising radiation, the electrons are swept towards the n-type material, and the holes are swept towards the p-type material.
Semiconductors can be used as detectors without being fabricated into diodes. For example, in a sodium-iodide detector, the ionising radiation also creates electron-hole pairs, but the electrons are permitted to fall back into the holes. As they do so, energy is released in the form of light, and the amount of light is counted by photomultiplier tubes. The amount of light detected by the photomultiplier tubes can then be used to infer the energy deposited in the sodium-iodide crystal by the ionising radiation.
No comments:
Post a Comment
Note: only a member of this blog may post a comment.